FDA issues an emergency permit for the use of remdesivir as COVID-19 treatment
The medication will be given to patients with coronavirus who are "hospitalized with severe disease"
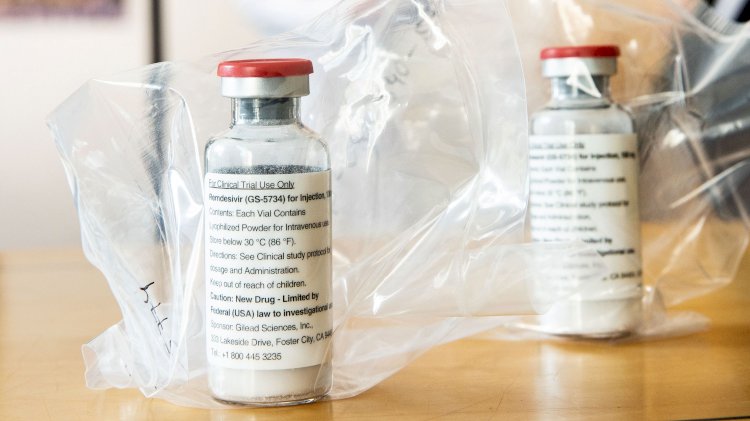
The FDA announced on the afternoon of 1 May 2020 that while information on the safety or efficacy of the experimental drug is still limited, a clinical trial earlier this week showed a faster recovery time for certain patients.
Remdesivir, an experimental antiviral drug developed by the pharmaceutical company Gilead Sciences, will be administered into suspected or confirmed coronavirus patients by health care providers in which the patient has low levels of blood oxygen, requires oxygen therapy or requires respiratory assistance such as a ventilator.
Secretary of Health and Human Services Alex Azar called it "a big move forward in the COVID-19 fight." Approval comes only two days after the drug showed "clear-cut positive impact" in a US study. The US National Institute for Allergy and Infectious Diseases said Wednesday that preliminary results from a US-based clinical trial indicated that it could help patients recover faster from coronavirus.
At that time, Dr. Anthony Fauci, director of NIAID, said the early findings were "a very valuable proof of concept because what it has shown is that a medication can block this virus." The NIAID trial involved 1,000 patients, showing a 31 % faster recovery time and a marginally lower mortality rate. Remdesivir was initially intended for Ebola treatment but was largely unsuccessful when trialled. A 2017 study showed it was successful against human coronavirus.
According to Gilead hospitals with ICUs and hospitals, the federal government agrees that obtaining its minimal supply of remdesivir would give priority to the drug's need most. "Gilead is working on the logistics of remdesivir delivery with the US Government and will provide more detail when the company begins shipping the product," the company said on 1 May 2020.
They will continue to collaborate with partners across the globe to expand our supply of remdesivir while advancing our ongoing clinical trials to complement our understanding of the drug's profile, àccording to Daniel O'Day, CEO of Gilead Sciences.





























